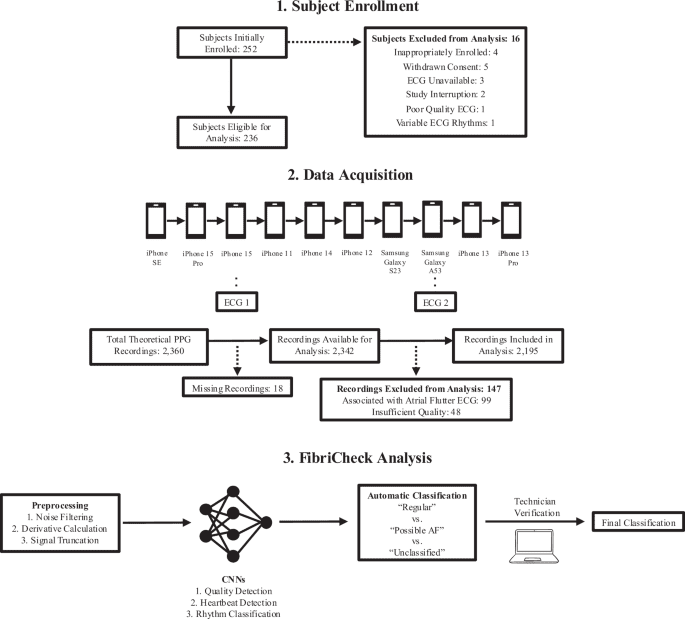
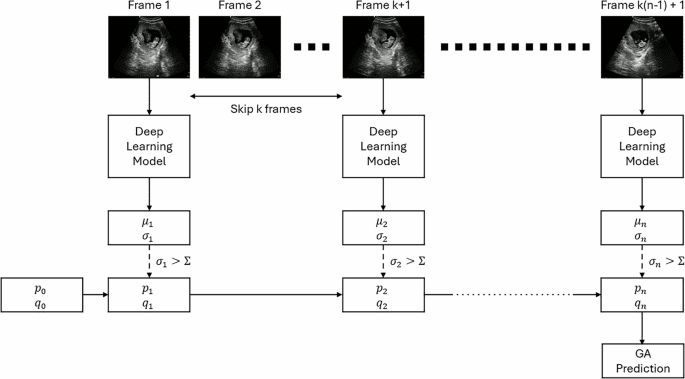
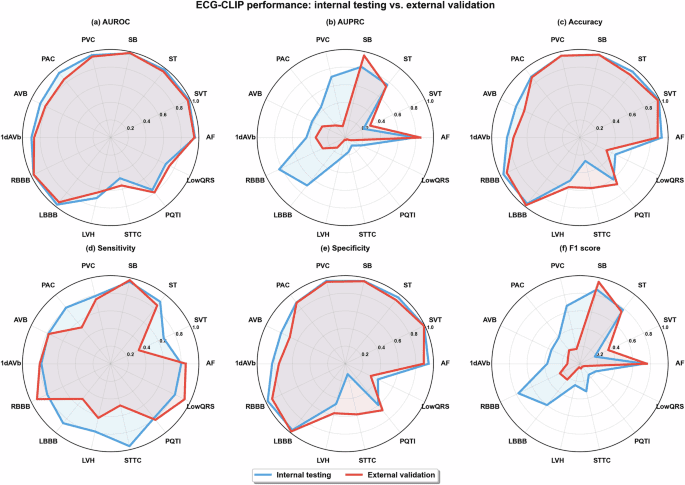
Obtaining Patient-Reported Outcome Data via a Home Patient Monitoring App: Development, Implementation, and Validation of Novel Interface Terminology
Background: Adverse events (AEs) related to cancer treatment represent a valuable source of information that can be used to adjust therapy for individual patients. The NIH developed the Common Terminology Criteria for Adverse Events…
Continue Reading