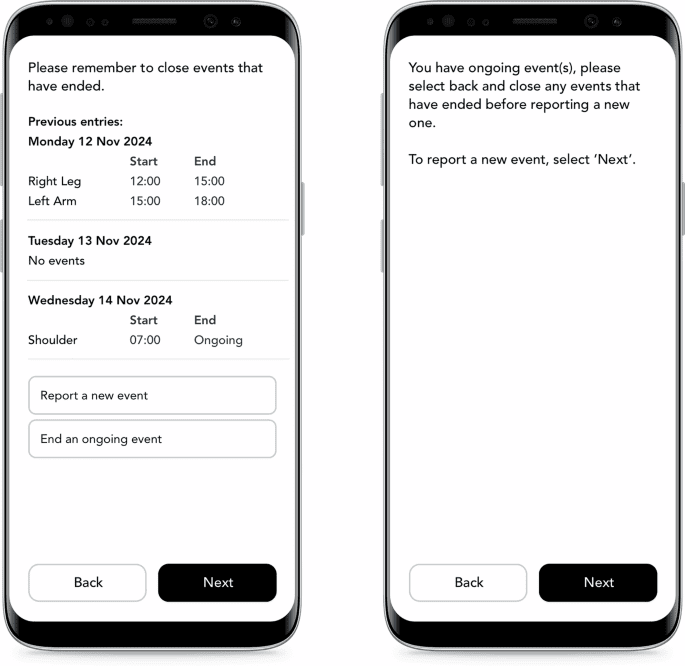
Best practice recommendations and considerations for designing and electronically implementing event-driven diaries in clinical trials
Based on the literature and collective experience and consensus of the authors, this article provides a definition for an event-driven electronic diary (EDeD), outlines considerations for the use of EDeDs, and provides best practice…
Continue Reading