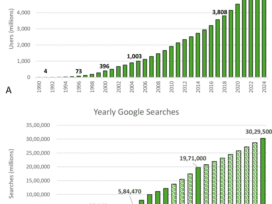
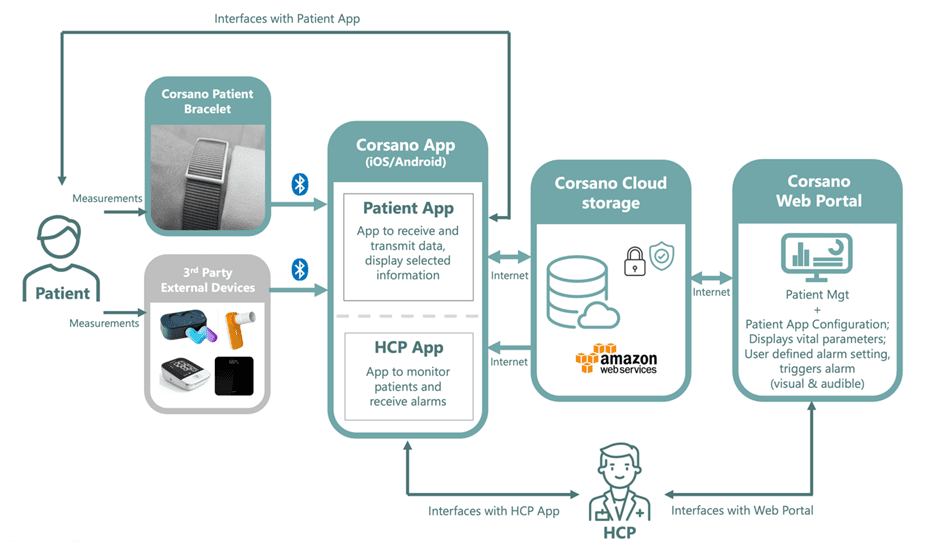
Philips Respironics Reaches Final Agreement with US Regulators on Sleep Apnea Device Recall
What You Should Know:
– Philips Respironics, a subsidiary of Royal Philips, has finalized a consent decree with the US Department of Justice (DOJ) and Food and Drug Administration (FDA) to address issues raised during…
Continue Reading