Revolutionary FDA Guidelines Transform AI Medical Devices
Groundbreaking AI Healthcare Regulations Unveiled
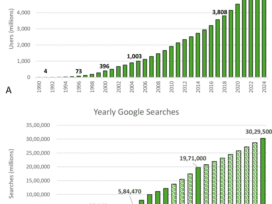
The U.S. Food and Drug Administration (FDA) has initiated a transformative step in healthcare technology by releasing comprehensive draft guidance for AI-enabled medical devices on January 6, 2025. This pioneering…