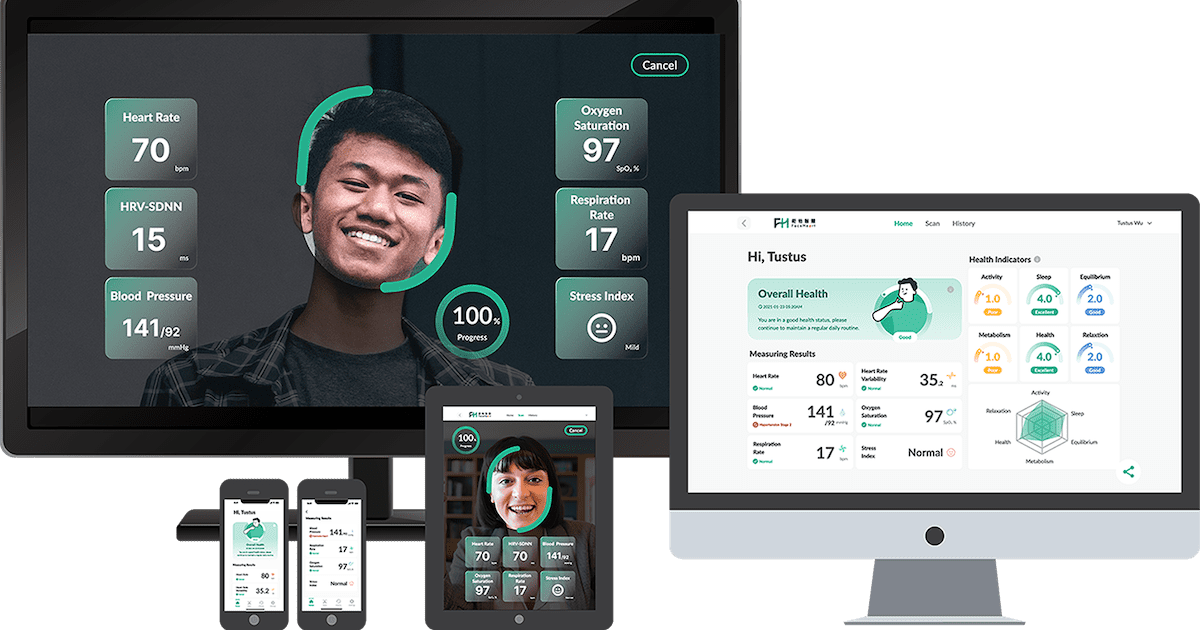
FDA Launches Elsa AI Technology Platform 2025
Revolutionary AI Platform Announcement
The U.S. Food and Drug Administration has officially launched Elsa, a groundbreaking generative FDA AI technology designed to revolutionize how agency employees perform their daily tasks. This innovative platform represents a…