Healthcare AI News 1/21/26
News
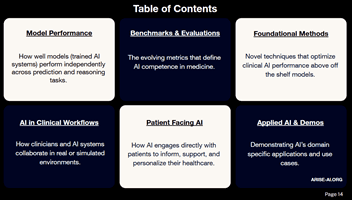
Authors from the ARISE academic medical center AI research network predict that 2026 will bring the first AI-related malpractice lawsuit, widespread rollout of urgent-care AI agents, and an escalating AI arms race between health…
Continue Reading