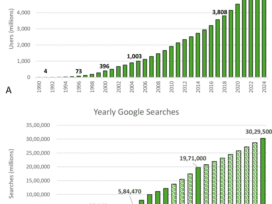
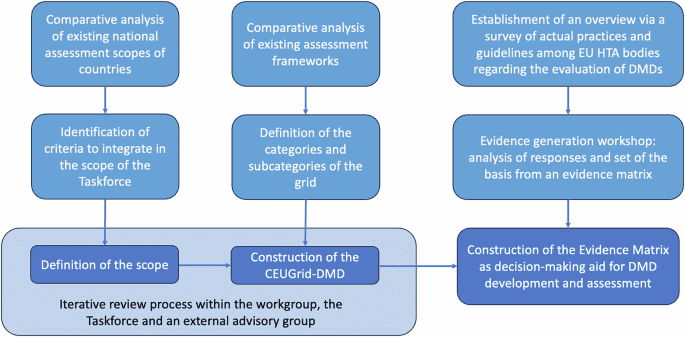
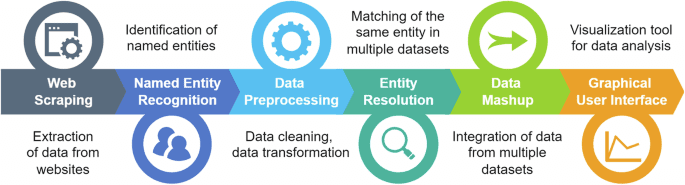
Industry Spotlight: Vanessa Counaert, Head of Connected Care for Western Europe, Baxter
Vanessa Counaert, head of connected care for Western Europe at Baxter (Credit: Baxter)
As healthcare organisations across Europe pursue digital transformation, Vanessa Counaert, head of connected care for Western Europe (WEU) at Baxter, highlights…
Continue Reading