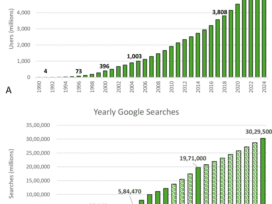
FDA Authorizes First Covid-19 and Flu Test, But Its Maker Files for Bankruptcy
Cough, fever, and breathing difficulty could signal a Covid-19 infection. It could also be the flu. A Lucira Health diagnostic is now the first one authorized by the FDA to identify the culprit virus…
Continue Reading