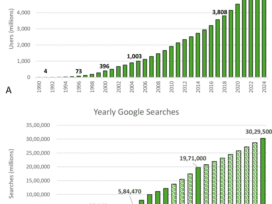
Reducing red-tape for healthcare organisations with Provider Connect Australia.
Visit www.digitalhealth.gov.au to see how we’re connecting Australia to a healthier future, and you to better healthcare.No audio? No problem. We've transcribed it below: When a new provider joined Longlife Medical, Mariana had to…
Continue Reading