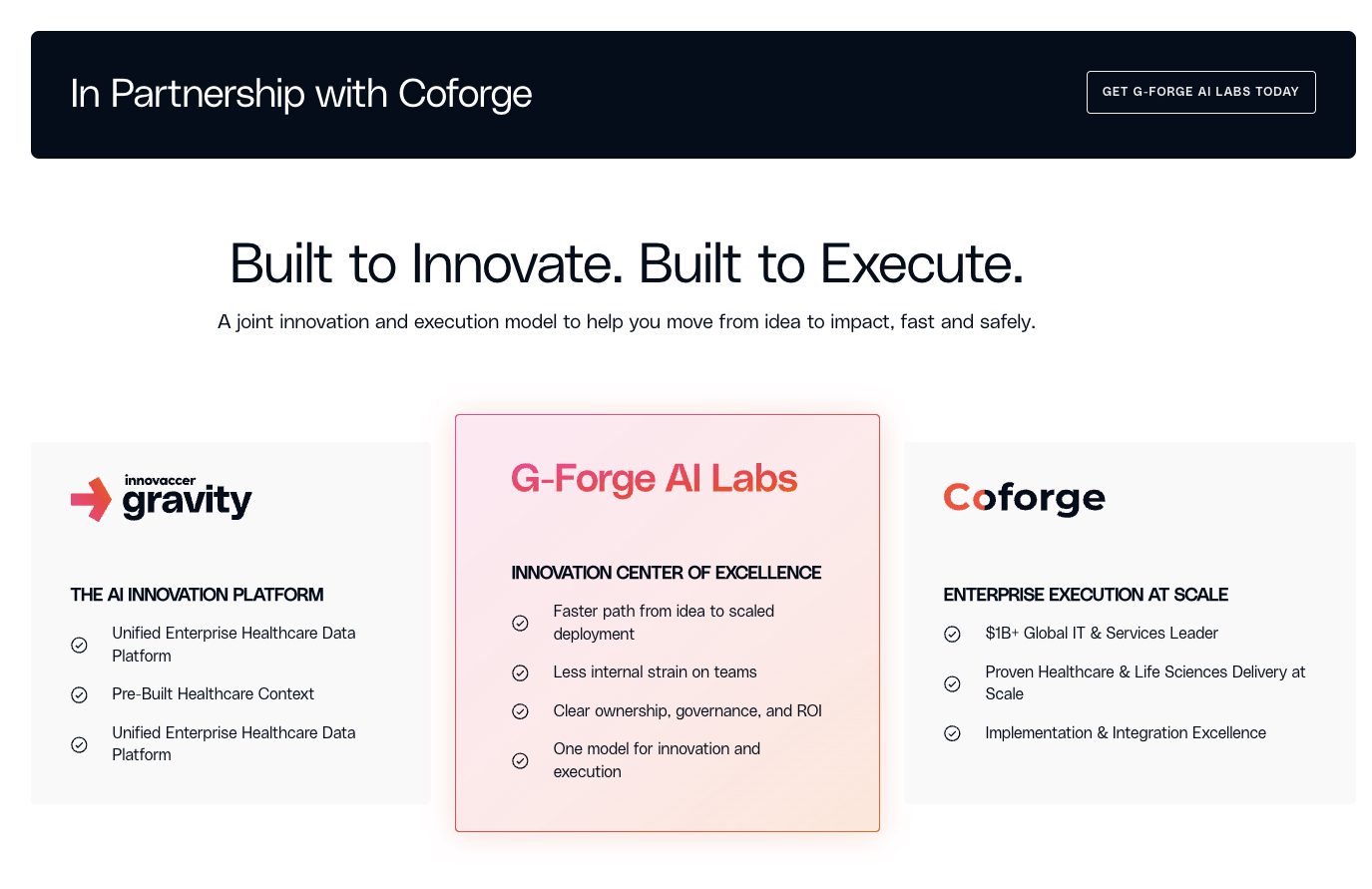
HGP 2026 Market Review: Why Two Decades of Health IT Failed to Bend the Cost Curve
What You Should Know
The Reality Check: In HGP’s 20-year retrospective, the verdict is harsh: technology delivered on workflows but failed on economics. Since HGP’s founding, U.S. healthcare spending has doubled to $5.3 trillion, proving…
Continue Reading